
Russian coronavirus vaccine: dangerous to administer or another anti-vaccination hysteria?
/ Главная / Russkiy Mir Foundation / Publications / Russian coronavirus vaccine: dangerous to administer or another anti-vaccination hysteria?Russian coronavirus vaccine: dangerous to administer or another anti-vaccination hysteria?
Russia has become the first country in the world to register a vaccine against coronavirus, but it seems that no one is happy about this. Many write that it is impossible to release a vaccine so quickly, because they will not have time to pass all the checks. Others describe it as a "massive human experiment." It is alleged that even before the third phase of clinical trials, Sputnik-V will be administered to millions of Russian citizens - in the coming month. It is reported that the new vaccine has many adverse reactions, but it allegedly provides few protective antibodies. Each of these theses is incorrect to one degree or another. However, this does not mean that the vaccine necessarily works. Let's try to figure out how things really are.

Photo credit: Defence Ministry press service
Let's start with the facts. They are as follows: The Gamaleya Research Institute of Epidemiology and Microbiologyhas indeed created a vaccine based on technology that is different from the ones used for all other coronavirus vaccines being developed on Earth today. And it is also true that it was registered as the first in the world - but not at all in the way most media in Russia and the Western countries write about it.
Well-known science and technology website ArsTechnica puts headline "Russia skips COVID-19 vaccine trial, says millions to be vaccinated this month." Similar errors can be seen in the publications of the Russian media: there are so many of them that it makes no sense to cite all of them here. Alas, this is an erroneous point of view.
Why the vaccine was registered
Such statement as "it is impossible to release medical products so quickly - they, by definition, will not have time to pass all the necessary checks." is based on a misunderstanding of the very process of developing vaccines in Russia.
In Russia, it is impossible to begin the third clinical phase of trials of a new vaccine without first receiving a temporary registration of such a vaccine from the Ministry of Health. Such registration is given after the first and second clinical phases, which only show whether the new vaccine is harmless, whether it poses significant health risks. The new vaccine has passed so far through only two phases: that is, it has not yet been tested to see if it helps to prevent contracting the virus. Volunteers were not kept in a place where it was highly likely to happen. The scientists only checked the level of antibodies to coronavirus and a number of other immune parameters.
But why was the registration announced in the first place if the widespread use of the vaccine "for millions" is still impossible? The answer to this question was given quite a long time ago in an interview with Denis Logunov, one of the key developers of Sputnik-V: "Registration under limited conditions is needed so that people from the risk group could participate in the study - we are not going to protect healthy volunteers with this vaccine."
That is, without such registration, the vaccine could be given only to the people whose age and health are such that they do not belong to the risk groups for coronavirus.
The meaning of the recent registration, therefore, was not to "become the first", as the media state. It is simply impossible to carry out the third phase of clinical trials without temporary registration. The stories about “registering to be the first” come simply from ignorance of how vaccines can be tested in Russia. Therefore, the confirmation of the state registration of the new vaccine is still to be done. And it will happen only after the third phase of clinical trials.
Despite this fact, the mass production of the vaccine is planned to be started in September. How is it possible if it is still undergoing trials? It's pretty simple.
First, Roszdravnadzor claims that 2,000 people should participate in the third phase of testing over three months. Tens of percent of these people must become infected with the coronavirus in vivo. But this is a theory: vaccinated people will not always face the virus in their daily lives, which means that the third phase may have to be extended. So, it's better to have more doses of the vaccine ready.
Secondly, and more importantly, today the developers of Sputnik-V are confident in it to such an extent (and the Ministry of Health as well) that they consider it necessary to distribute the vaccine to as many people from risk groups as possible even before the end of the third phase.
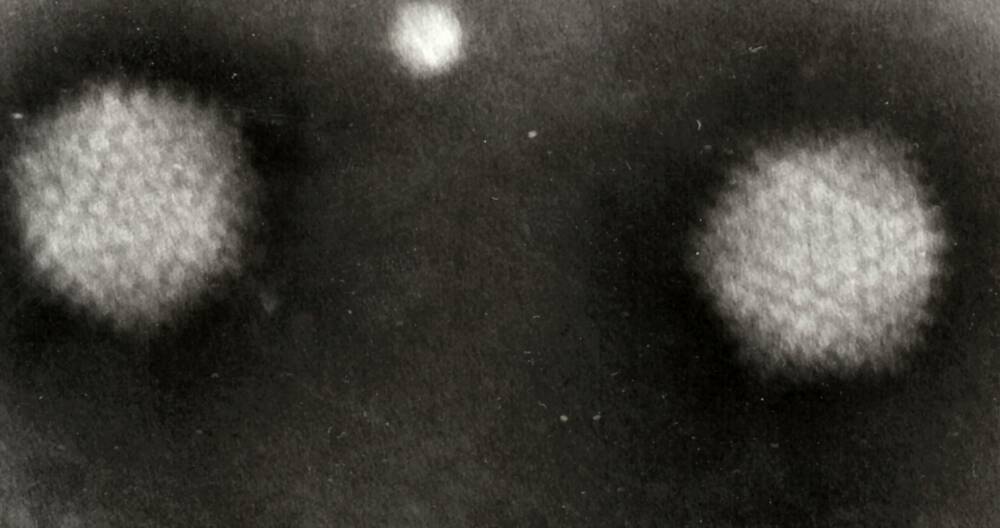
Photo: Adenovirus type-5 vectored COVID-19 vaccine developed by Sputkik-V. It consists of two types of human adenovirus (not one like in Chinese analogue)
To whom and when the new vaccine to be delivered?
About the risk groups. Undoubtedly, doctors are among them. As the pandemic showed both around the world and in Russia, the percentage of cases and deaths among doctors is higher than in the population as a whole. It is for them that the mass production of the vaccine will start in September. It will be administered voluntarily and - since we are talking about doctors – by the most informed part of society about the possibilities of the vaccine.
The vaccination will begin only for doctors at the end of August, and it is not included in the third phase of clinical trials. After all, this third phase includes only those who are constantly monitored - completely, in all respects, which will be difficult for tens and hundreds of thousands of doctors (and then teachers). It is about them that the CEO of the Russian Direct Investment Fund (RDIF) Kirill Dmitriev. The fund, which has financed the development and production of the vaccine, claims that "tens of thousands of volunteers will be vaccinated within a month." Note the difference: not "millions this month", as in the Western media puts it, but tens of thousands during the month - that is, including part of September.
Can this be regarded as a "great experiment on humans"? Is it unethical? It is hard to say. If vaccination is really voluntary (as it is now declared), then certainly not. Experimental vaccination usually involves not doctors and teachers, but people of all professions. In theory, a doctor should understand better than a regular volunteer what a vaccine is and whether they want to use it. Teachers, too, must be able to make decisions as well as the whole population.
The second most important risk group is the elderly people. Today the vaccine is recommended only for people between 18 and 60 years old. This is not for some disadvantages that have been identified for older people. This is because the first and second phases of clinical trials were conducted on two groups of 38 people aged from 18 to 60. The reasons for age restrictions are clear: older people are traditionally given new vaccines only after making sure they do not cause health problems in younger people.

Boris Johnson claims that once developed the British vaccine will go to the British citizens first. The Sputnik-V is in different position.
The vaccine is to be brought abroad in certain quantities even for the clinical trials. (Photo credit: Flickr/Financial Times)
In fact, the vaccine has already been administered to people over 60. There are many of them among the staff of the Gamaleya Center itself - including its head, Academician Gintsburg (he is under 70). Despite his age, he underwent vaccinations well. However, this does not mean that it will be administered en masse to elderly people without testing.
Just after August 11, 2020, the so-called "post-marketing phase" of vaccine research began. As noted by the same Ginzburg, one of the goals in this case is "to study the possibility of its use in terms of safety and immunogenicity for people over 60 years old, even for 90 years old." If all goes well in about three months Sputnik-V may begin to be given to people over 60.
However, those who are under the age of 18 won’t get the vaccine so quickly. According to the law, the third phase of testing on them goes by age groups, and from 8 to 18 there are three such groups. Each of the groups is, in fact, a separate phase within the third phase, and each will take at least three months. This means that for the majority of children, permission to administer the vaccine to be received only next year. Fortunately, children are the least threatened group.
Strange as it may seem, many foreign citizens will be the first to receive the vaccine. UAE, Saudi Arabia, the Philippines, Brazil and a number of other countries have already expressed their desire to participate in the third phase of clinical trials of Sputnik-V.
Why are the developers so confident in the safety of the vaccine? What about tons of side effects?
Many media wrote that the vaccine caused a vast number of adverse events - either 144 or 200 per 38 volunteers. The official sources (Russian instructions for the use of the vaccine) show a slightly different picture: there were 175 adverse events among 38 volunteers. Of these, 144 passed in 42 days of observation, and 31 did not pass, although in four out of 31 cases the cases the situation was improving.
It may seem that more than four adverse events per person vaccinated is a lot. It is alarming that there should be means for anti-shock therapy in places for vaccination. Famous bloggers generally describe the situation simply: registering such a vaccine is a crime.
Unfortunately, bloggers don’t know enough on the matter. To begin with, about anti-shock therapy: according to the standards of the Ministry of Health, means for it should be in every room where vaccination is carried out: not only against a new virus, but also any other. Now for the side effects.
To understand whether something is safe or not, it must be compared with alternatives. There is another vaccine vaguely similar to the Russian vaccine in the third phase of clinical trials - a British vaccine based on a modified chimpanzee adenovirus. Sputnik-V uses a modified human adenovirus, but the difference is not that big. How many side effects does the British counterpart have that has not yet received registration?
According to the Lancet, Phase I trials of the British vaccine uses other methods to calculate the incidence of adverse events. But even they give a clear picture: fatigue and headache were typical for most patients. Tens of percent experienced pain at the injection point and general weakness, 60% of those who received the vaccine experienced muscle pain, 61% reported general mild illness, 56% reported chills, 51% fever, 18% of volunteers who did not take antipyretic showed a temperature of at least 38 ° C, and 2% - of 39 ° C and above.
As we can see, in the Russian-speaking users of social networks believe in high-quality imported vaccines much more than Western peer-reviewed scientific journals. According to the Lancet and the developers of the British vaccine themselves, side effects in their recipients are not one in a million, and not one in a thousand - on the contrary, this is the norm. But, as in the case of the Russian counterpart, there were no serious health consequences - even people with 39 ° C experienced such a temperature for a short time, without any long-term troubles.

Photo: Daily2dailynews.com title
Where does such confidence comes from?
Ok, everything is clear with the side effects – they are present in both cases, the frequency is comparable. But another question arises. Why are the Gamaleya Center so confident in Sputnik-V that they are already offering doctors vaccination? What allows Ginzburg, the head of the center, to believe that immunity from the vaccine will last two years, since only a few months have passed since the start of testing?
The thing is that the new Russian vaccine can only be called one by stretching a point. The very approach of vaccination against diseases using the adenovirus "engine" has been developed at the Gamaleya Center since the 1990s, and more than one vaccine has been made on it.
The latest example of this kind is the Russian Ebola vaccine tested in West Africa. The adenovirus vaccines against it have long been registered and have passed all three phases of clinical trials. 2,000 people participated in the third phase in Africa, that is, in terms of scope, it is the same as planned in Russia for coronavirus.
The essence of such a vaccine and its differences from foreign analogues are quite simple. In the human body and a number of other animals, a common adenovirus is found, which causes symptoms that we call the “cold” in everyday life. You can take one of the types of such adenoviruses and deprive it of its ability to reproduce in a person (through genetic modification) so that it cannot give the infected person cold symptoms.
The adenoviral approach has one theoretical weakness. It is believed that if a person is ill with an adenovirus, then his immunity can cope with the adenovirus vaccine so effectively that it simply will not have time to “train” him to fragments of another virus. In the West, this is seen as the main problem with the Chinese adenovirus vaccine CanSino. It uses a type 5 adenovirus, Ad5, as a carrier of a fragment of the coronavirus. 20-50% of the world's population is immune to this virus, which means that it will be more difficult to protect such people with the new Chinese vaccine.

At the Gamaleya Center, two types of adenovirus carriers are used at once: with the first dose of the vaccine, those made on the basis of Ad5 are introduced, and with the second - those based on Ad26. Geographically, these adenoviruses are found in different zones, and people rarely have simultaneous immunity against both types of viruses. Tests of such a vaccine against Ebola have proved it: it forms a fairly strong immunity, which, judging by clinical trials in West Africa, lasts two years.
That is why Academician Ginzburg, who heads the center, said that the new vaccine against coronavirus could work for two years: the delivery agent plays an important role in the effectiveness of the vaccine, and in Sputnik-V he is exactly the same as in earlier two-dose vaccines by the Gamaleya center.
The British vaccine based on adenovirus differs sharply from the Russian and Chinese ones. It uses a chimpanzee adenovirus, ChAdOx1. In theory, this is good: people do not have immunity from it, the body will not have time to "deal" with it before the delivery virus "acquaints" our immune system with the coronavirus.
But the problem is that so far not a single vaccine based on the chimpanzee adenovirus has received registration (in contrast to the Gamaleya vaccine). This means that the UK product test cycle must be longer if necessary. After all, this is not another product based on a spent carrier made from human adenoviruses, as in Russia or China. Therefore, it can be said with a high degree of certainty that the British vaccine is unlikely to be mass-produced in 2020.
The Gamaleya center expects to reach production levels of 3-5 million doses of Sputnik-V per month by the end of 2020. However, we are not quite sure that it will be that simple.
Why we need to separate vaccines from politics
Reactions to the vaccine in Russia (and not only) have become politicized, and therefore many react to it according to the principle: since it was done so quickly, it means that they were in a hurry for political reasons, that means it is dangerous.
As we have shown above, this is not the case: the vaccine was registered, first of all, because without temporary registration it simply cannot be fully tested. The fact that, despite this, it will be offered to doctors and teachers is hardly dangerous, because the bulk of the material introduced with the vaccine has long passed clinical trials. Its new component (encoding a coronavirus protein) simply cannot make the vaccine really dangerous for a healthy person.
This is not the result of some wonderful medicine in our country. It's just that the Gamaleya Center was lucky: they had a ready-made vaccine based on the human adenovirus, into which the coronavirus protein was quickly introduced with minimal changes.
Maybe it was better to wait?
But why are we in such a hurry? It is clear that clinical trials are needed. But why give the opportunity to vaccinate doctors and teachers? What if they all rush to get vaccinated, and then they have health problems?
It is known that hundreds of thousands of people have died from the coronavirus. It is known that not a single vaccine has resulted in even thousands of deaths or long-term damage to health in the last 100 years. This is despite the fact that most mass vaccines were created many decades ago, at a time of much less stringent clinical trial standards.
What is more important to us? The probable possibility of saving tens of thousands of lives, or the unlikely risk of harm from a vaccine that can hardly be called new?
Of course, there will always be those who choose not to get vaccinated. But we would advise you to pay attention to one little thing: vaccine developers know much more about them than antivaccination activists. However, in the case of the Gamaleya Center, its staff, including the head of the center, have already been vaccinated. Perhaps Academician Ginzburg and Doctor of Biological Sciences Logunov know some details that have escaped the attention of bloggers? Future will tell.

Photo credit: Фото: Gustavo Fring / Pexels
Will the vaccine be effective?
We showed the reasons why 69-year-old Ginzburg calmly injected himself with the "new" Sputnik-V vaccine: there is not much new in it and that is why it is safe. Its adenovirus "working horse" has long gone through all phases of clinical trials in its anti-ebolovirus version. And hasn't shown any serious or long-term side effects.
All that is really new in it is the gene that encodes the coronavirus protein. By itself, this protein does not pose a noticeable danger to humans, it is simply physically too small for it to be able to do anything other than “acquaint” our immunity with the coronavirus. In addition, a short time after vaccination, it disappears from the body and cannot have a long-term effect on it.
But this only means that the new vaccine is harmless. Whether it will also be useful is another question. Unfortunately, there is little data here. The journalists already rushed to write that, as if, the level of antibodies that neutralize coronavirus after the new vaccine is low, only 49.3.
The problem is that it certainly isn't. The level of antibodies capable of neutralizing a particular virus and sufficient to protect against it is always different. No one has yet done a massively successful coronavirus vaccination.
Therefore, it is completely unknown whether the level of 49.3 neutralizing antibodies will be enough to prevent a person from contracting such a virus. To make this clear, the third phase of clinical trials is needed - one in which people will be exposed to the risk of infection, which so far, in the first and second phases of clinical trials, simply did not exist.
It is likely that Sputnik-V should help some of the vaccinated. This is indicated by the fact that antibodies were found in all 100% of the volunteers after the vaccine.
But for protection against coronavirus, cellular immunity can also play a significant role, and its parameters after vaccination have not yet been published. As a result, it is impossible to say in advance what percentage of those who received the Russian vaccine will not get sick, until the end of the third phase of clinical trial.
Therefore, from a scientific point of view, it is simply too early to predict the success of this particular vaccine. Whether it will protect 15% or 95% of its recipients is still completely unknown. And it is unlikely that it will be reliably known earlier than November this year. We would not hope so strongly that it will become a panacea for the new disease. As Naked Science wrote back in the spring, there are cases when it is not possible to create proper vaccines for viral diseases for decades.
But one thing should be understood: there is a demand for the vaccine, and it is very high. In June 2020, excess mortality in Russia was 25,500 people. And although according to Rosstat, only 12,000 of these people were infected with coronavirus, there are serious reasons to suspect that the rest of them simply could not be identified. The reason is that after the first days of illness, there may no longer be a coronavirus on a smear from the throat (it has already "gone down").
Therefore, it can be assumed that just in June 2020, the coronavirus in Russia killed a quarter of 100,000 people. This is a lot: as a result, the overall mortality rate in the country soared by more than 18%. In autumn, conditions for the spread of coronavirus will be much better than June.
New publications

 Mikhail Kalatozov, a director who transformed the world of cinematography in many ways, was born 120 years ago. He was a Soviet film official and a propagandist. Above all, he was capable of producing movies that struck viewers with their power and poetic language.
Mikhail Kalatozov, a director who transformed the world of cinematography in many ways, was born 120 years ago. He was a Soviet film official and a propagandist. Above all, he was capable of producing movies that struck viewers with their power and poetic language.  Ukrainian authorities have launched a persecution campaign against the canonical Ukrainian Orthodox Church (UOC), the biggest one in the country's modern history. Over the past year, state sanctions were imposed on clergy representatives, searches were conducted in churches, clergymen were arrested, criminal cases were initiated, the activity of the UOC was banned in various regions of the country, and monasteries and churches were seized.
Ukrainian authorities have launched a persecution campaign against the canonical Ukrainian Orthodox Church (UOC), the biggest one in the country's modern history. Over the past year, state sanctions were imposed on clergy representatives, searches were conducted in churches, clergymen were arrested, criminal cases were initiated, the activity of the UOC was banned in various regions of the country, and monasteries and churches were seized.  When Nektary Kotlyaroff, a fourth-generation Russian Australian and founder of the Russian Orthodox Choir in Sydney, first visited Russia, the first person he spoke to was a cab driver at the airport. Having heard that Nektariy's ancestors left Russia more than 100 years ago, the driver was astonished, "How come you haven't forgotten the Russian language?" Nektary Kotlyaroff repeated his answer in an interview with the Russkiy Mir. His affinity to the Orthodox Church (many of his ancestors and relatives were priests) and the traditions of a large Russian family brought from Russia helped him to preserve the Russian language.
When Nektary Kotlyaroff, a fourth-generation Russian Australian and founder of the Russian Orthodox Choir in Sydney, first visited Russia, the first person he spoke to was a cab driver at the airport. Having heard that Nektariy's ancestors left Russia more than 100 years ago, the driver was astonished, "How come you haven't forgotten the Russian language?" Nektary Kotlyaroff repeated his answer in an interview with the Russkiy Mir. His affinity to the Orthodox Church (many of his ancestors and relatives were priests) and the traditions of a large Russian family brought from Russia helped him to preserve the Russian language.

 The leaders of the Friends of the Great Russia cultural association (Amici Della Grande Russia) in Italy believe that the Western policy of abolishing Russian culture in Europe has finally failed. Furthermore, it was doomed to failure from the beginning.
The leaders of the Friends of the Great Russia cultural association (Amici Della Grande Russia) in Italy believe that the Western policy of abolishing Russian culture in Europe has finally failed. Furthermore, it was doomed to failure from the beginning.  Name of Vladimir Nemirovich-Danchenko is inscribed in the history of Russian theater along with Konstantin Stanislavski, the other founding father of the Moscow Art Theater. Nevertheless, Mr. Nemirovich-Danchenko was a renowned writer, playwright, and theater teacher even before their famous meeting in the Slavic Bazaar restaurant. Furthermore, it was Mr. Nemirovich-Danchenko who came up with the idea of establishing a new "people's" theater believing that the theater could become a "department of public education."
Name of Vladimir Nemirovich-Danchenko is inscribed in the history of Russian theater along with Konstantin Stanislavski, the other founding father of the Moscow Art Theater. Nevertheless, Mr. Nemirovich-Danchenko was a renowned writer, playwright, and theater teacher even before their famous meeting in the Slavic Bazaar restaurant. Furthermore, it was Mr. Nemirovich-Danchenko who came up with the idea of establishing a new "people's" theater believing that the theater could become a "department of public education."  "Russia is a thing of which the intellect cannot conceive..." by Fyodor Tyutchev are famous among Russians at least. December marks the 220th anniversary of the poet's birth. Yet, he never considered poetry to be his life's mission and was preoccupied with matters of a global scale. Mr.Tyutchev fought his war focusing on relations between Russia and the West, the origins of mutual misunderstanding, and the origins of Russophobia. When you read his works today, it feels as though he saw things coming in a crystal ball...
"Russia is a thing of which the intellect cannot conceive..." by Fyodor Tyutchev are famous among Russians at least. December marks the 220th anniversary of the poet's birth. Yet, he never considered poetry to be his life's mission and was preoccupied with matters of a global scale. Mr.Tyutchev fought his war focusing on relations between Russia and the West, the origins of mutual misunderstanding, and the origins of Russophobia. When you read his works today, it feels as though he saw things coming in a crystal ball...